Publication Spotlights
Prefusion-stabilized SARS-CoV-2 S2-only antigen provides protection against SARS-CoV-2 challenge
Hsieh Ching-Lin, Leist Sarah R., Miller Emily Happy, Zhou Ling, Powers John M., Tse Alexandra L., Wang Albert, West Ande, Zweigart Mark R., Schisler Rohit K., Chandran Kartik, Baric Ralph S., McLellan Jason S.
Nat Commun. 2024 Feb 20; 15:1553. PMID: 38378768
Ever-evolving SARS-CoV-2 variants of concern (VOCs) have diminished the effectiveness of therapeutic antibodies and vaccines. Developing a coronavirus vaccine that offers a greater breadth of protection against current and future VOCs would eliminate the need to reformulate COVID-19 vaccines. Here, we rationally engineer the sequence-conserved S2 subunit of the SARS-CoV-2 spike protein and characterize the resulting S2-only antigens. Structural studies demonstrate that the introduction of interprotomer disulfide bonds can lock S2 in prefusion trimers, although the apex samples a continuum of conformations between open and closed states. Immunization with prefusion-stabilized S2 constructs elicits broadly neutralizing responses against several sarbecoviruses and protects female BALB/c mice from mouse-adapted SARS-CoV-2 lethal challenge and partially protects female BALB/c mice from mouse-adapted SARS-CoV lethal challenge. These engineering and immunogenicity results should inform the development of next-generation pan-coronavirus therapeutics and vaccines.
Vaccination with prefusion-stabilized respiratory syncytial virus fusion protein elicits antibodies targeting a membrane-proximal epitope
McCool RS, Musayev M, Bush SM, Derrien-Colemyn A, Acreman CM, Wrapp D, Ruckwardt TJ, Graham BS, Mascola JR, McLellan JS.
J Virol. 2023 Sep 22:e0092923. PMID: 37737588
Respiratory syncytial virus (RSV) is the leading cause of bronchiolitis and pneumonia in infants, infecting 90% of all children by age 2. Fusion of the RSV virion with a host cell is facilitated by the fusion protein (F). Previous work showed that immunization with a stabilized RSV F antigen elicited a diverse set of neutralizing antibodies. Here, we further investigated a group of these antibodies, known as public clonotype 2 (PC2), that appeared to be more prevalent in immunized individuals as compared to naturally infected individuals. Structural and biophysical characterizations revealed that PC2 members target the novel antigenic Site VI, despite their initial classification as Site IV binders based on patch mutation studies. We similarly investigated an antibody with high sequence similarity to PC2 members that was derived from a naturally infected adult. Initial yeast display-based competition assays suggested this antibody targeted site Ø; however, our structural studies revealed this antibody instead targets Site VI. These findings suggest that either Site VI antibodies were frequently misidentified or that soluble ectodomain vaccines enhance Site VI targeting by removing steric hindrance introduced by the viral membrane. In either case, this work has meaningful implications for how we analyze the humoral response to antigens and how those antigens themselves are rationally designed.
Structural Basis for Binding of Neutralizing Antibodies to Clostridioides difficile Binary Toxin
Goldsmith JA, Dewar V, Hermand P, Blais N, McLellan JS
Bacteriol. 2023 Apr 25;205(4):e0045622. PMID: 36951574
Clostridioides difficile is a gram-positive opportunistic human pathogen that causes 15,000 deaths annually in the United States, prompting a need for vaccine development. In addition to the important toxins TcdA and TcdB, binary toxin (CDT) plays a significant role in pathogenesis of certain C. difficile ribotypes by catalyzing the ADP-ribosylation of actin in host cells. However, the mechanisms of CDT neutralization by antibodies have not been studied, limiting our understanding of key epitopes for CDT antigen design. Therefore, we isolated neutralizing monoclonal antibodies against CDT and characterized their mechanisms of neutralization structurally and biochemically. 2.5 Å- and 2.6 Å-resolution X-ray crystal structures of the antibodies BINTOXB/22 and BINTOXB/9, respectively, in complex with CDTb—the CDT subunit that forms a heptameric pore for the delivery of toxic CDTa enzyme into the host cytosol—showed that both antibodies sterically clash with adjacent protomers in the assembled heptamer. Assessment of trypsin-induced oligomerization of the purified CDTb protoxin in vitro showed that BINTOXB/22 and BINTOXB/9 prevented the assembly of di-heptamers upon prodomain cleavage. This work suggests that the CDT oligomerization process can be effectively targeted by antibodies, which will aid in the development of C. difficile vaccines and therapeutics.
Structural basis for antibody recognition of vulnerable epitopes on Nipah virus F protein
Byrne PO, Fisher BE, Ambrozak DR, Blade EG, Tsybovsky Y, Graham BS, McLellan JS* and Loomis RJ*
Nat Commun. 2023 Mar 17;14(1):1494. PMID: 36932063
Nipah virus (NiV) is a pathogenic paramyxovirus that causes mild to severe disease in humans, sometimes progressing to fatal encephalitis. Two envelope glycoproteins, the attachment protein (G) and fusion protein (F), facilitate entry into host cells. During cell entry, NiV F rearranges from a compact prefusion conformation to an extended postfusion conformation. Prefusion F elicits higher neutralizing antibody titers than postfusion F in mice, indicating the prefusion form as a target for development of vaccines and therapeutics. Several neutralization-sensitive epitopes on the NiV F apex have been described, however the antigenicity of most of the F protein’s surface remains uncharacterized. Here, we immunize mice with prefusion-stabilized NiV F and isolate ten monoclonal antibodies that neutralize pseudotyped virus. Cryo-electron microscopy reveals eight neutralization-sensitive epitopes on NiV F, four of which have not previously been described. Novel sites span the lateral and basal faces of NiV F, expanding the known library of vulnerable epitopes. Seven of ten antibodies bind the Hendra virus (HeV) F protein. Multiple sequence alignment suggests that some of these newly identified neutralizing antibodies may also bind F proteins across the Henipavirus genus. This work identifies new epitopes as targets for therapeutics, provides a molecular basis for NiV neutralization, and lays a foundation for development of new cross-reactive antibodies targeting Henipavirus F proteins.
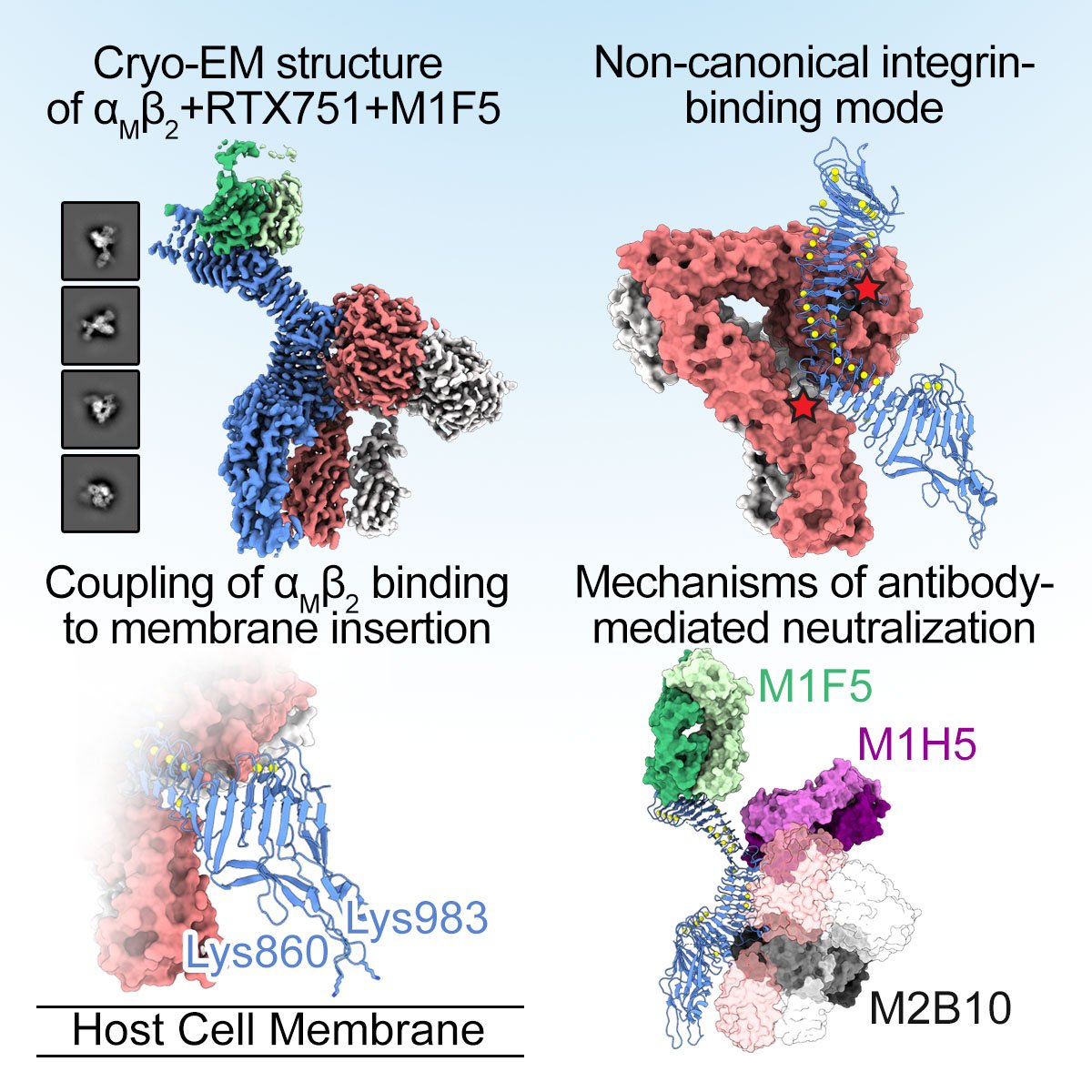
Integrins are ubiquitous cell-surface heterodimers that are exploited by pathogens and toxins, including leukotoxins that target β2 integrins on phagocytes. The Bordetella adenylate cyclase toxin (ACT) uses the αMβ2 integrin as a receptor, but the structural basis for integrin binding and neutralization by antibodies is poorly understood. Here we use cryo-electron microscopy to determine a 2.7 Å resolution structure of an ACT fragment bound to αMβ2. This structure reveals that ACT interacts with the headpiece and calf-2 of the αM subunit in a non-canonical manner specific to bent, inactive αMβ2. Neutralizing antibody epitopes map to ACT residues involved in αM binding, providing the basis for antibody-mediated attachment inhibition. Furthermore, binding to αMβ2 positions the essential ACT acylation sites, which are conserved among toxins exported by type I secretion systems, at the cell membrane. These findings reveal a structural mechanism for integrin-mediated attachment and explain antibody-mediated neutralization of ACT intoxication.
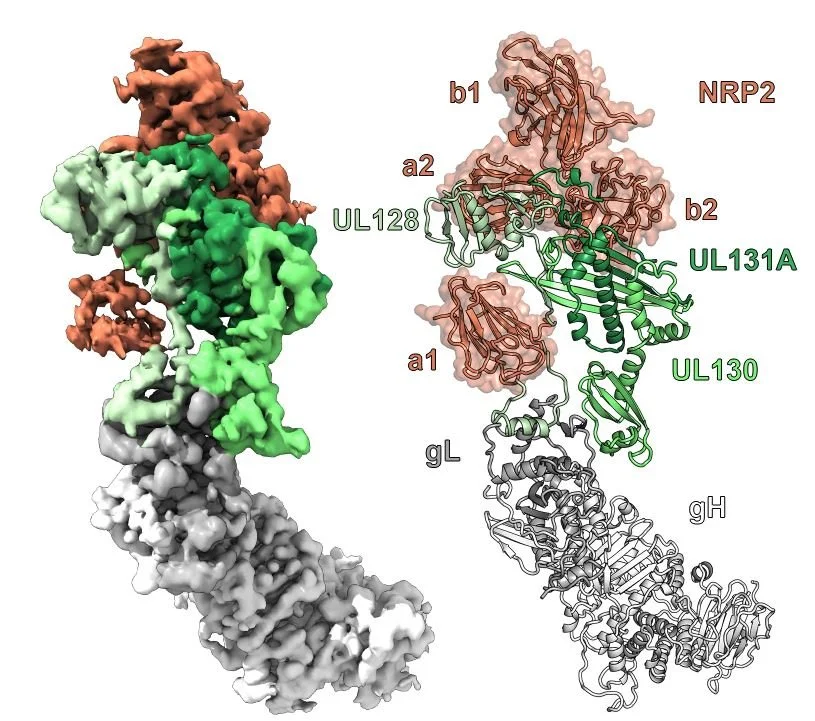
Structural basis for HCMV Pentamer recognition by neuropilin 2 and neutralizing antibodies
Wrapp D, Ye X, Ku Z, Su H, Jones HG, Wang N, Mishra AK, Freed DC, Li F, Tang A, Li L, Jaijyan DK, Zhu H, Wang D, Fu TM, Zhang N, An Z, McLellan JS.
Sci Adv. 2022 Mar 11;8(10):eabm2546.
Human cytomegalovirus (HCMV) encodes multiple surface glycoprotein complexes to infect a variety of cell types. The HCMV Pentamer, composed of gH, gL, UL128, UL130, and UL131A, enhances entry into epithelial, endothelial, and myeloid cells by interacting with the cell surface receptor neuropilin 2 (NRP2). Despite the critical nature of this interaction, the molecular determinants that govern NRP2 recognition remain unclear. Here, we describe the cryo-EM structure of NRP2 bound to Pentamer. The high-affinity interaction between these proteins is calcium dependent and differs from the canonical carboxyl-terminal arginine (CendR) binding that NRP2 typically uses. We also determine the structures of four neutralizing human antibodies bound to the HCMV Pentamer to define susceptible epitopes. Two of these antibodies compete with NRP2 binding, but the two most potent antibodies recognize a previously unidentified epitope that does not overlap the NRP2-binding site. Collectively, these findings provide a structural basis for HCMV tropism and antibody-mediated neutralization.
Structural basis of synergistic neutralization of Crimean-Congo hemorrhagic fever virus by human antibodies
Mishra AK, Hellert J, Freitas N, Guardado-Calvo P, Haouz A, Fels JM, Maurer DP, Abelson DM, Bornholdt Z, Walker LM, Chandran K, Cosset FL, McLellan JS, Rey FA.
Science. 2022 Jan 7;375(6576):104-109.
Crimean-Congo hemorrhagic fever virus (CCHFV) is the most widespread tick-borne zoonotic virus, with a 30% case fatality rate in humans. Structural information is lacking for the CCHFV membrane fusion glycoprotein Gc—the main target of the host neutralizing antibody response—as well as for antibody–mediated neutralization mechanisms. We describe the structure of prefusion Gc bound to the antigen-binding fragments (Fabs) of two neutralizing antibodies that display synergy when combined, as well as the structure of trimeric, postfusion Gc, in collaboration with Félix Rey’s group at Institut Pasteur. The structures show the two Fabs acting in concert to block membrane fusion, with one targeting the fusion loops and the other blocking Gc trimer formation. The structures also revealed the neutralization mechanism of previously reported antibodies against CCHFV, providing the molecular underpinnings essential for developing CCHFV–specific medical countermeasures for epidemic preparedness.
Potent neutralization of SARS-CoV-2 variants of concern by an antibody with an uncommon genetic signature and structural mode of spike recognition
Kramer KJ, Johnson NV, Shiakolas AR, …, Crowe JE Jr, Bukreyev A, Carnahan RH, McLellan JS, Georgiev IS.
Cell Rep. 2021 Oct 5;37(1):109784.
Current therapeutic antibody treatments for SARS-CoV-2 have been shown to lose efficacy against variants of concern (VOCs). Therefore, continued efforts to identify broadly reactive monoclonal antibodies against SARS-CoV-2 are necessary to develop lasting effective interventions. In collaboration with the Geogiev lab at Vanderbilt University, we screened the B-cell repertoire of a patient previously infected with SARS-CoV-2 for neutralizing antibodies using the linking B cell receptor to antigen specificity through sequencing (LIBRA-seq) technology. Our lead therapeutic candidate, antibody 54042-4, was shown to be potently neutralizing against authentic SARS-CoV-2 viruses, including VOCs. Our structure of 54042-4 bound to the SARS-CoV-2 spike revealed an epitope that is composed of residues that are highly conserved in currently circulating SARS-CoV-2 lineages. These findings demonstrate the potential of 54042-4 as an effective therapeutic candidate for current and future VOCs.
Selected Publications
Goldsmith JA, DiVenere AM, Maynard JA, McLellan JS.
Structural basis for antibody binding to adenylate cyclase toxin reveals RTX linkers as neutralization-sensitive epitopes
PLoS Pathog. 2021 Sep 21;17(9):e1009920.
PMID: 34547035
Mukhamedova M*, Wrapp D*, Shen CH*, Gilman MSA*, Ruckwardt TJ, Schramm CA, Ault L, Chang L, Derrien-Colemyn A, Lucas SAM, Ransier A, Darko S, Phung E, Wang L, Zhang Y, Rush SA, Madan B, Stewart-Jones GBE, Costner PJ, Homan LA, Hickman SP, Berkowitz NM, Doria-Rose NA, Morabito KM, DeKosky BJ, Gaudinski MR, Chen GL, Crank MC, Misasi J, Sullivan NJ, Douek DC, Kwong PD, Graham BS, McLellan JS, Mascola JR.
Vaccination with prefusion-stabilized respiratory syncytial virus fusion protein induces genetically and antigenically diverse antibody responses
Immunity 2021 Apr 13;54(4):769-780.e6.
PMID: 33823129
Hsieh CL, Goldsmith JA, Schaub JM, DiVenere AM, Kuo HC, Javanmardi K, Le KC, Wrapp D, Lee AG, Liu Y, Chou CW, Byrne PO, Hjorth CK, Johnson NV, Ludes-Meyers J, Nguyen AW, Park J, Wang N, Amengor D, Lavinder JJ, Ippolito GC, Maynard JA, Finkelstein IJ, & McLellan JS.
Structure-based design of prefusion-stabilized SARS-CoV-2 spikes
Science 2020 369(6510):1501-1505
PMID: 32703906
Wrapp D, De Vlieger D, Corbett KS, Torres GM, Wang N, Van Breedam W, Roose K, van Schie L, VIB-CMB COVID-19 Response Team, Hoffmann M, Pöhlmann S, Graham BS, Callewaert N, Schepens B, Saelens X, McLellan JS
Structural Basis for Potent Neutralization of Betacoronaviruses by Single-Domain Camelid Antibodies
Cell 2020 81(6):1436-1441
PMID: 32375025
Wrapp D, Wang N, Corbett KS, Goldsmith JA, Hsieh C-L, Abiona O, Graham BS, McLellan JS.
Cryo-EM structure of the 2019-nCoV spike in the prefusion conformation
Science 2020 367(6483):1260-1263
PMID: 32075877
Gilman MSA, Liu C, Fung A, Behera I, Jordan P, Rigaux P, Ysebaert N, Tcherniuk S, Sourimant J, Eléouët J-F, Sutto-Ortiz P, Decroly E, Roymans D, Jin Z, McLellan JS.
Structure of the respiratory syncytial virus polymerase complex
Cell 2019 179(1):193-204. Full text
PMID: 31495574
Wang N, Rosen O, Wang L, Turner HL, Stevens LJ, Corbett KS, Bowman CA, Pallesen J, Shi W, Zhang Y, Leung K, Kirchdoerfer RN, Becker MM, Denison MR, Chappell JD, Ward AB, Graham BS, McLellan JS.
Structural definition of a neutralization-sensitive epitope on the MERS-CoV S1-NTD
Cell Rep. 2019 28(13):3395-3405. Full text PDF
PMID: 31553909
Gilman MSA, Furmanova-Hollenstein P, Pascual G, van ‘t Wout AB, Langedijk JPM, McLellan JS.
Transient opening of trimeric prefusion RSV F proteins
Nat Commun. 2019 10(1):2105. Full text PDF
PMID: 31068578
Jones HG, Ritschel T, Pascual G, Brakenhoff JPJ, Keogh E, Furmanova-Hollenstein P, Lanckacker E, Wadia JS, Gilman MSA, Williamson RA, Roymans D, van 't Wout AB, Langedijk JP, McLellan JS.
Structural basis for recognition of the central conserved region of RSV G by neutralizing human antibodies
PLoS Pathog. 2018 14(3):e1006935.
PMID: 29509814
Goodwin E, Gilman MSA, Wrapp D, Chen M, Ngwuta JO, Moin SM, Bai P, Sivasubramanian A, Connor RI, Wright PF, Graham BS, McLellan JS, Walker LM.
Infants infected with respiratory syncytial virus generate potent neutralizing antibodies that lack somatic hypermutation
Immunity 2018; 48(2):339-349.e5.
PMID: 29396163
Battles MB, Más V, Olmedillas E, Cano O, Vázquez M, Rodríguez L, Melero JA, McLellan JS.
Structure and immunogenicity of prefusion-stabilized human metapneumovirus F glycoprotein
Nat Commun. 2017 Nov 16;8(1):1528.
PMID: 29142300
Tian D, Battles MB, Moin SM, Chen M, Modjarrad K, Kumar A, Kanekiyo M, Graepel KW, Taher NM, Hotard AL, Moore ML, Zhao M, Zheng ZZ, Xia NS, McLellan JS, Graham BS.
Structural basis of respiratory syncytial virus subtype-dependent neutralization by an antibody targeting the fusion glycoprotein
Nat Commun. 2017 Nov 30;8(1):1877.
PMID: 29187732
Pallesen J, Wang N, Corbett KS, Wrapp D, Kirchdoerfer RN, Turner HL, Cottrell CA, Becker MM, Wang L, Shi W, Kong WP, Andres EL, Kettenbach AN, Denison MR, Chappell JD, Graham BS, Ward AB, McLellan JS.
Immunogenicity and structures of a rationally designed prefusion MERS-CoV spike antigen
PNAS 2017 Aug 29;114(35):E7348-E7357.
PMID: 28807998
Rossey I, Gilman MS, Kabeche SC, Sedeyn K, Wrapp D, Kanekiyo M, Chen M, Mas V, Spitaels J, Melero JA, Graham BS, Schepens B, McLellan JS, Saelens X.
Potent single-domain antibodies that arrest respiratory syncytial virus fusion protein in its prefusion state
Nat Commun. 2017 Feb 13;8:14158.
PMID: 28194013
Gilman MS, Castellanos CA, Chen M, Ngwuta JO, Goodwin E, Moin SM, Mas V, Melero JA, Wright PF, Graham BS, McLellan JS, Walker LM.
Rapid profiling of RSV antibody repertoires from the memory B cells of naturally infected adult donors
Sci Immunol. 2016 Dec 16;1(6). pii: eaaj1879.
PMID: 28111638
Misasi J, Gilman MS, Kanekiyo M, Gui M, Cagigi A, Mulangu S, Corti D, Ledgerwood JE, Lanzavecchia A, Cunningham J, Muyembe-Tamfun JJ, Baxa U, Graham BS, Xiang Y, Sullivan NJ, McLellan JS.
Structural and molecular basis for Ebola virus neutralization by protective human antibodies
Science. 2016 Mar 18;351(6279):1343-6.
PMID: 26917592
Kirchdoerfer RN, Cottrell CA, Wang N, Pallesen J, Yassine HM, Turner HL, Corbett KS, Graham BS, McLellan JS, Ward AB.
Pre-fusion structure of a human coronavirus spike protein
Nature. 2016 Mar 3;531(7592):118-21.
PMID: 26935699
Battles MB, Langedijk JP, Furmanova-Hollenstein P, Chaiwatpongsakorn S, Costello HM, Kwanten L, Vranckx L, Vink P, Jaensch S, Jonckers TH, Koul A, Arnoult E, Peeples ME, Roymans D, McLellan JS.
Molecular mechanism of respiratory syncytial virus fusion inhibitors
Nat Chem Biol. 2016 Feb;12(2):87-93.
PMID: 26641933
Krarup A, Truan D, Furmanova-Hollenstein P, Bogaert L, Bouchier P, Bisschop IJ, Widjojoatmodjo MN, Zahn R, Schuitemaker H, McLellan JS, Langedijk JP.
A highly stable prefusion RSV F vaccine derived from structural analysis of the fusion mechanism
Nat Commun. 2015 Sep 3;6:8143
PMID: 26333350
Gilman MS, Moin SM, Mas V, Chen M, Patel NK, Kramer K, Zhu Q, Kabeche SC, Kumar A, Palomo C, Beaumont T, Baxa U, Ulbrandt ND, Melero JA, Graham BS, McLellan JS.
Characterization of a prefusion-specific antibody that recognizes a quaternary, cleavage-dependent epitope on the RSV fusion glycoprotein
PLoS Pathog. 2015 Jul 10; 11(7):e1005035.
PMID: 26161532